Abstract
Introduction. Ruxolitinib (RUX) is a JAK1/2 inhibitor that may control myelofibrosis (MF)-related splenomegaly and symptoms and can be prescribed regardless of age. Aging correlates with clinically impaired prognosis probably due to biologically increased molecular complexity. However, assessment of efficacy/safety of RUX and correlation with clonal architecture in elderly MF patients (pts) is relatively unknown.
Aims. To report the clinical outcome of 277 pts with WHO-defined MF treated with RUX when aged ≥65 yrs. To correlate molecular status assessed by targeted sequencing of 30 genes known to be involved in myeloid disorders with response to therapy and survival.
Methods. A clinical database was created in 20 European Hematology Centers. Retrospective data on 487 MF pts treated with RUX according to prescribing information from Jan 2011 to Jun 2016 were collected. Spleen (SR) and Symptoms (SyR) Response to RUX were evaluated according to the 2013 IWG-MRT criteria. Survival was calculated from the start of RUX. Event-free survival (EFS) included progression to acute leukemia (AL), death and RUX discontinuation. Peripheral blood (PB) granulocytes-derived DNA was extracted and processed according to the "Myeloid Solution" (SOPHIA GENETICS) assay on the MiSeq Illumina platform. Variants were pre-classified as potentially pathogenic by SOPHiATM Artificial Intelligence. Output data was analyzed by Sophia DDM v3 software.
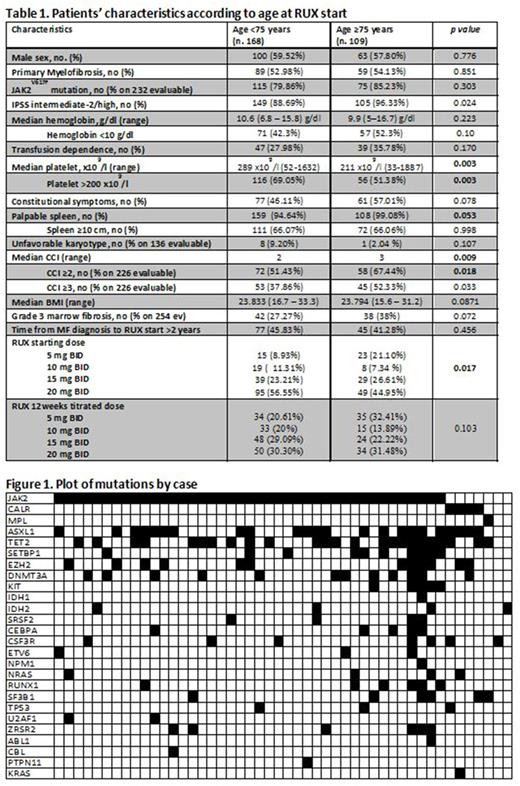
Results. A total of 277 out of 487 pts (46.6%) started RUX when aged ≥65 yrs. Compared to elderly (age 65-74) pts, very elderly (≥ 75 yr) pts had a higher Charlson Comorbidity Index and a lower platelet count, thus started RUX at lower doses (Table 1). At 6 mos, SR and SyR were achieved by 35.9% and 83.7% of 209 and 221 evaluable pts, respectively. In the first 6 mos, anemia/thrombocytopenia/infections ≥G2 were observed in 33.6%, 21.7% and 29.2% of the 277 pts, respectively. The only feature independently associated with increased infectious risk was a history of previous infection. Evolution into AL occurred in 22 pts. Overall, 110 pts discontinued RUX after a median time of 12.5 mos. Importantly, the rate of toxicities, responses, and disease evolution was not influenced by age. After a median follow-up of 19.5 mos, 65 pts died because of progression of myelofibrosis (40%), heart disease (10.8%), infections (13.8%), AL (15.4%), hemorrhage/thrombosis (7.7%) or transplant-associated toxicity (4.6%). Older age and Body Mass Index <21 (first quartile) was significantly associated with worse survival (p=0.02).
NGS will be performed on 100/277 pts with available PB samples at RUX start. Preliminary data on 48 elderly pts (median age, 72.5 yrs) showed that all pts had a mutation in ≥1 gene (Fig. 1). A total of 523 variants have been identified so far. Only 297 (57%) variants were considered potentially pathogenetic. Four genes (BRAF, FLT3, HRAS, WT1) investigated in the NGS panel were never mutated. Recurrent mutations with potential pathogenic impact included TET2 (20,5%), ASXL1 (14%), SETBP1 (7,7%), EZH2 (6,7%), DNMT3A (4,7%), KIT (3,7%). The percentages of pts with pathogenetic variants for the most recurrently mutated genes were: ASXL1 (50%), TET2 (50%), DNMT3A (22,9%), EZH2 (25%), SETBP1 (21%), KIT (10,4%). At least one high molecular risk (HMR) pathogenetic mutation was present in 61% of pts; 37% had ≥2 HMR mutations. The most frequently mutated HMR genes were ASXL1 and EZH2, that co-occurred in 22% of the cases. HMR status was significantly associated with PMF and larger (>10 cm) spleen length (p=0.04). Additionally, patients with EZH2 mutation and ≥3 variants showed a lower baseline hemoglobin and platelet count (respectively p=0.04 and p=0.03). Molecular status (HMR, ≥2 HMR mutations, ≥3 variants and ASXL1- or EZH2-mutations) was investigated for association with clinical responses/outcome parameters. Preliminary data showed a trend for association of HMR+ and ≥3 variants with worse EFS and leukemia-free survival.
Discussion. Since response/toxicity rates were not influenced by age, older age should not be a limitation for RUX therapy. The assessment of comorbidities, nutrition status and infectious history may identify specific pts fragilities. The cohort of molecularly annotated pts will be further enriched. However, we have so far observed a high molecular complexity that did not seem to affect responses and survival in the elderly.
LC and FHH equally contributed.
Bonifacio: Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Abruzzese: Novartis: Consultancy; Pfizer: Consultancy; BMS: Consultancy; Incyte: Consultancy. Tiribelli: Incyte: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Breccia: Bristol Myers Squibb: Consultancy; Novartis: Consultancy; Incyte: Consultancy; Pfizer: Consultancy. Cavazzini: Pfizer: Consultancy; Incyte: Consultancy; Bristol-Meyers Squibb: Consultancy; Novartis: Consultancy. Crugnola: Celgene: Honoraria; BMS: Honoraria; Novartis: Honoraria. Martinelli: Amgen: Consultancy; Celgene: Consultancy; Pfizer: Consultancy; Ariad/Incyte: Consultancy; Johnson&Johnson: Consultancy; Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal